IVD Disposables
Plastics engineered for accuracy, biocompatibility, and manufacturing precision.
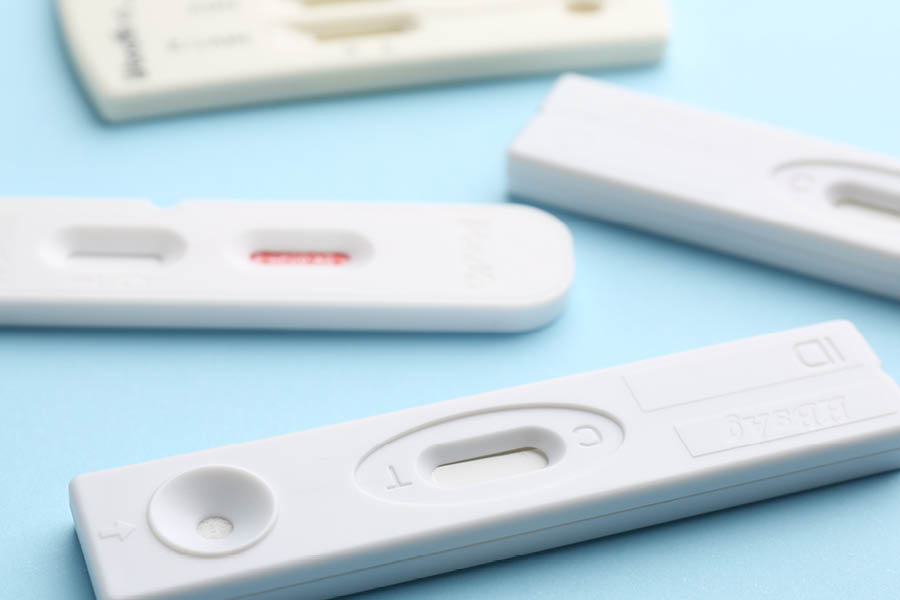
From point-of-care to clinical labs, Americhem’s polymer solutions for diagnostic consumables are engineered for clarity, consistency, and compliance. Our compounds support fast cycle manufacturing and critical performance in:
From manufacturing to final application, our materials help protect integrity and accuracy.
Americhem formulations are designed to meet global standards like ISO 10993-5 and USP Class VI, backed by documentation and validation support.
Plastics engineered for accuracy, biocompatibility, and manufacturing precision.
Compounds with clarity, chemical resistance, and durability for consumer testing products.
High-purity formulations for centrifuge tubes, pipette tips, and diagnostic labware.
Portable testing requires reliable materials, ensuring repeatable, accurate diagnostics.

Our healthcare grade polymer solutions are formulated for consistency, patient safety, and global market requirements.

Flexible, compliant compounds that adapt to rapid changes in diagnostic technology.
Americhem supports diagnostic device OEMs and contract manufacturers with polymer materials designed for processability and regulatory confidence. Whether you’re scaling rapid tests or launching a next-gen IVD platform, we support your journey with:
Americhem materials are tested to meet FDA, ISO 10993-5, USP Class VI, and REACH requirements for medical-grade plastics.

Real materials. Real timelines. Real results. Partner with a team that understands regulatory needs and delivers polymer solutions that perform in the real world.